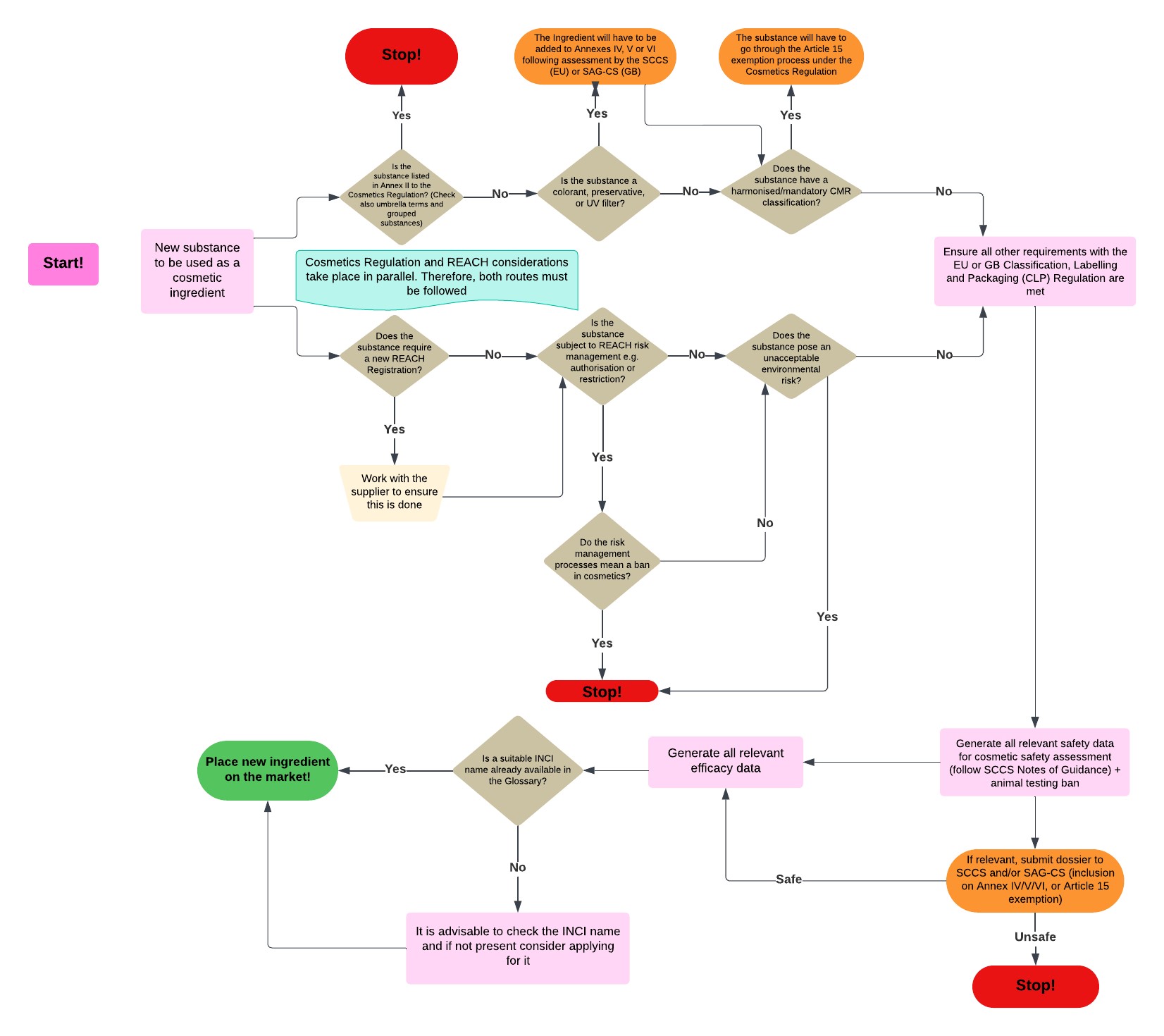
The new cosmetic ingredient must comply with all applicable laws. This guidance only refers to the following chemicals legislation: Cosmetics Regulation; Classification, Labelling and Packaging (CLP) Regulation; Registration, Evaluation and Authorisation of Chemicals (REACH) Regulation.
However, companies need to review whether there are any other pieces of legislation; for example, environmental, sourcing, licensing, or others, which apply to the new ingredient.
The below flowchart can be downloaded here.