The Classification, Labelling and Packaging (CLP) Regulation aims to ensure a high level of protection of health and the environment. The CLP Regulation is based on the United Nations’ Globally Harmonised System (GHS). CLP operates in both the UK and EU; however, the systems are independent and chemicals must comply with both legislations depending on the market of interest, as they are managed separately under both UK and EU CLP.
It is important to highlight that EU CLP applies to the EU Member States, EEA countries and Northern Ireland (in accordance with the Windsor Framework). GB CLP only applies to Great Britain (England, Wales and Scotland).
CLP requires manufacturers, importers or downstream users of substances or mixtures to classify, label and package chemicals appropriately before placing them on the market. The regulation applies to ALL substances and mixtures irrespective of the volume supplied.
Finished cosmetic products (ready for sale to the consumer) are exempt from the requirements of the GB CLP Regulation. However, cosmetic ingredients as raw materials or cosmetic mixtures (such as bulk of a cosmetic that is not in the finished state) fall under the remit of the GB CLP Regulation.
The European Chemicals Agency (ECHA) is responsible for CLP in the EU/NI; the Health and Safety Executive (HSE) regulates and enforces the GB CLP Regulation.
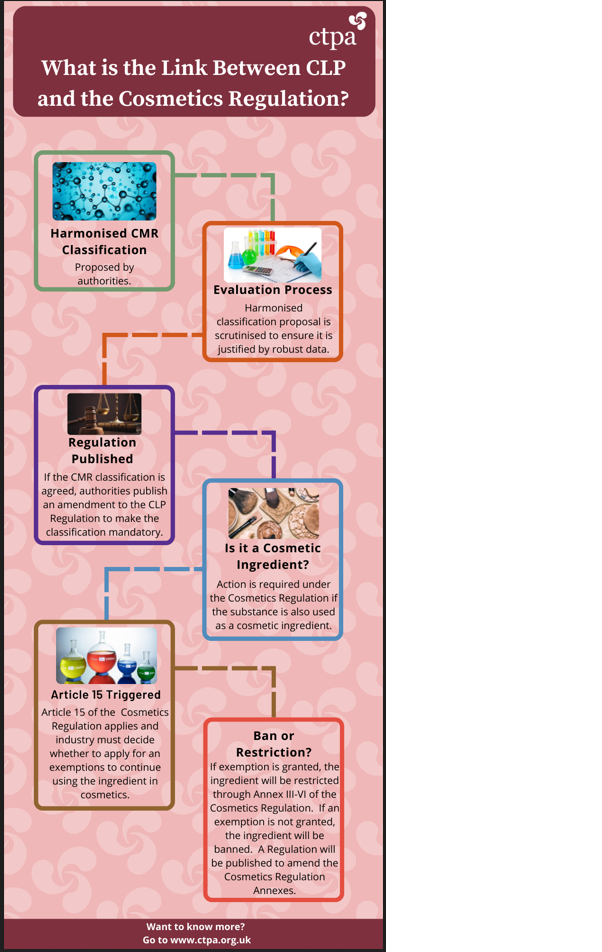
The below infographics outline what is the CLP Regulation, and explain its interaction with the Cosmetics Regulation.